At Fortrea, we know oncology drug development is inherently complex. Advancing the next generation of oncology therapeutics requires special considerations across the entire spectrum—from early phase oncology clinical trials to late stage and commercialization of a therapy and relevant diagnostic assays.
Read more on our website
Case Study: San Francisco Bay Area biotech advances phase I-II cancer immunotherapy trial with Fortrea
A biotech company approached Fortrea to manage a phase I-II clinical trial evaluating a personalized neo-antigen-based vaccine. The immunotherapy was tested in patients with NSCLC, colorectal cancer and gastroesophageal cancer. With support from Fortrea, this complex study was a success, and the novel immunotherapy was able to advance to phase II-III trials.

Article: Combining multiple therapies for metastatic breast cancer: ADCs And ICIs

Publication List: Recent Fortrea Publications about Antibody-Drug Conjugates
Antibody-drug conjugates (ADCs) represent one of the largest growth segments in oncology, holding the promise of more effective therapies that can offer lower systemic toxicity, for better patient outcomes.- Download this list of our top three recent publications about ADCs to learn more about how Fortrea oncologists are collaborating with others in the industry to accelerate advancements in ADC trial design.
Article: Advance your antibody-drug conjugate pipeline with our experience and expertise
ADCs are driving advances in oncology therapies, with more than a dozen treatments approved by the FDA and many more in the pipeline. Download our infographic that summarizes ADCs in the industry plus our experience in supporting clinical trials of ADCs.
Recent Peer-Reviewed Journal Articles 2020-2024;
Our Fortrea oncologists often author or co-author peer-reviewed journal articles. This collection of recent articles spans across the oncology clinical research landscape.
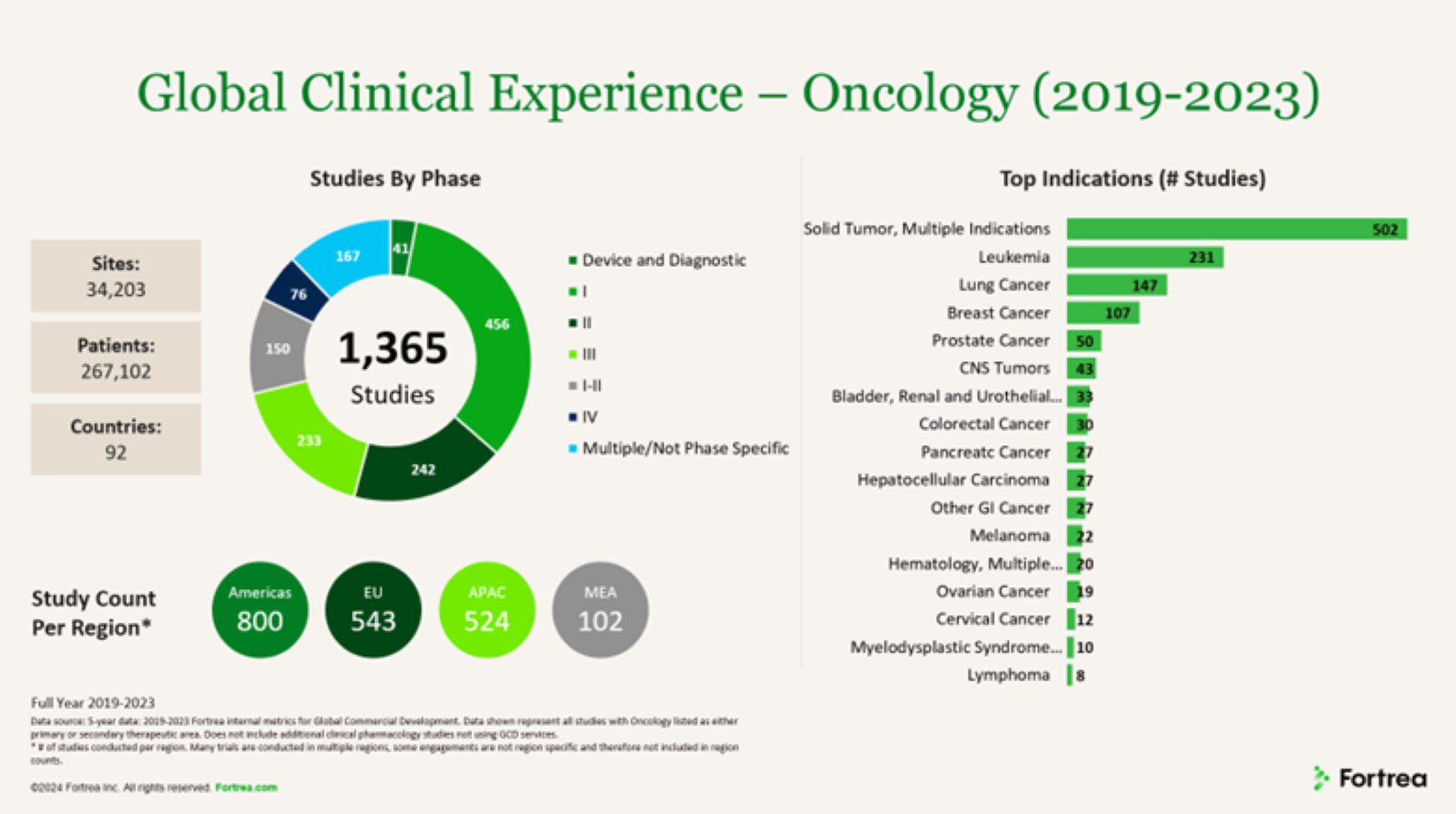
Fortrea’s oncology team has supported 1,365 studies across more than 34,000 investigator sites in the past 5 years
Case Study: Pediatric Oncology Rare Disease Trial
Download this case study that discusses the challenges of conducting a pediatric rare disease trial across several regions globally. In this trial, pediatric patients with a specific type of solid tumor and a rare genetic mutation were studied. Adding to the challenge were COVID-19-related site closures and restrictions. While work continues, the first two cohorts are fully enrolled and the program is on track!

Publication List: Recent Fortrea Immuno-oncology Publications
Immuno-oncology therapies, also known as cancer immunotherapies, represent a broad class of treatments such as targeted antibodies, tumor-infecting viruses, checkpoint inhibitors and cancer vaccines. The many types of therapeutic agents have been under study for decades and Fortrea has participated in over 290 immuno-oncology trials in just the last five years (2019-2023).
Recent Webinar
Accelerating Clinical Development through Design, Diversity & Digitalization in Oncology and Beyond
This dynamic panel-format webinar will explore how to speed drug development and improve return on investment (ROI) by adopting a “3-D” approach to trials focusing on Design, Diversity, and Digitalization. Oncology trials will be the focal point for our discussions, but the key insights are applicable to a wide range of therapeutic areas. The need to improve geographical, racial, ethnic, and other forms of diversity and inclusion in trials –including new legislation and regulatory guidance documents to spur adoption of diverse trials - has been at the forefront for several years. Insights will be shared on what has worked and what still needs to be done, and how new tools, digital and mobile health and other innovative approaches can lead the way.
With so many recent advances in applying “big data” strategies and Artificial Intelligence to trial design and execution, the speakers will discuss concrete ways these novel approaches have impacted trials and pipelines. Case studies and aggregate insights will be reviewed with an eye towards improving trial outcomes and performance against KPIs.
Enabling sites to do what sites do best
Fortrea places Sites and investigators at the forefront of trial planning and operations, working together to strengthen trial delivery and expand healthcare options for all.

Become a Fortrea site
At Fortrea, we want to change the sponsor-CRO-Site relationship forever. With our dedicated team, we have both the insights, experience, scale and processes to build the partnerships needed to get new treatments to patients faster. And we’d like you to join us.